News
Green alchemy: Turning greenhouse gases into hard carbon
Global warming and its dramatic effects are no longer a distant dream of the future, but a harsh reality. As the world grapples with rising temperatures and their destructive consequences, scientists are feverishly searching for solutions to combat rising greenhouse gas emissions and minimise their impact. One promising approach in this battle is called negative emission technologies (NETs), which aim to remove climate-changing CO2 directly from the atmosphere and store it safely. But how exactly does this work and what makes these technologies so special?

The rise of NETs
Negative emission technologies range from biological processes that convert CO2 into biomass to advanced chemical and electrochemical processes that convert CO2 into long-term stable materials such as hard carbon. These technologies offer a glimmer of hope because they can not only capture CO2 effectively, but also convert it into useful products.
The challenge of CO2 storage
However, storing CO2 in the form of oxalates or other stable materials is not without its challenges. Many of the existing methods require high pressure, special chemical reagents and are technically complex. In addition, the processes need to be sustainable and viable on a large scale to have a significant impact on reducing global CO2 emissions.
Breakthrough with cerium dioxide
A recent discovery has focused attention on one particular material: Cerium dioxide (CeO2), which has the ability to electrochemically convert CO2 into hard carbon. This discovery could be a game changer, as hard carbon is versatile and the conversion offers a direct way to remove CO2 from the atmosphere.
Using computer modelling to analyse the CeO2-CO2 reaction
To understand how CeO2 andCO2 interact, scientists at the University of Ulm carried out computer calculations using a method called density functional theory (DFT). They used special mathematical formulae to calculate the forces between the atoms more accurately and made additional adjustments (called Hubbard U) to further improve the accuracy, especially for the properties of CeO2. However, they encountered difficulties when trying to include the effects of liquids in their simulations, which is important in the real world. These problems could not be solved, so as a first step they decided to assume that the reactions only took place in gaseous form. This simplification is common and still provides useful information about the reactions.
Importance of pKa values for CO2 conversion
Researchers have recognised that the behaviour of CO2 in different solvents, in particular the acid dissociation constants (pKa values), is crucial for its effective conversion. Especially in non-aqueous solvents such as DMF - a common solvent in CO2 reduction - new calculation methods have to be used to accurately determine the pKa values.
What makes CeO2 special
CeO2 surfaces show their true strength in catalytic reactions especially in the so-called (110) crystal orientation. Here, the surface tends to form oxygen defects - small gaps that prove to be perfect docking sites for CO2. At certain voltages, up to 25% of the oxygen atoms on the surface can be removed, making CeO2 an ideal candidate for CO2 reduction.
The innovative route from CO2 to solid carbon
It all begins with the start of the chain, where CO2 is activated by binding to oxygen vacancies on the surface of CeO2. This first bond changes the chemical structure of CO2 and makes it ready to form carbon chains. In the next step, chain extension, the activated CO2 couples up with other molecules to form longer chains. This creates new carbon-carbon bonds from two CO2 molecules. The subsequent chain branching enables the formation of larger and more complex structures such as rings, which serve as the basis for further reactions. Chain branching also allows the formation of three-dimensional structures, paving the way for solid materials. CeO2 plays a key role not only at the start of the process, but also in stabilising the growing carbon structures.
Summary
The latest research results, funded by the German Federal Ministry of Education and Research and also supported by the Dr Barbara Mez-Starck Foundation, shed light on the role of CeO2 as a catalyst in the reduction of carbon dioxide (CO2) and its conversion into hard carbon. The results show that CeO2 acts mainly as an anchor for the initial adsorption of CO2. Interestingly, the subsequent reaction - including the oligomerisation and polymerisation steps - is largely independent of the catalyst. This mechanism opens up new possibilities for the use of CeO2 in CO2 reduction and could lead to the development of improved catalysts.
The research also shows that the rich defect chemistry of CeO2 provides ideal conditions for the adsorption of CO2, a crucial first step in the conversion process.
Call for research
Given the potential importance of these findings for the conversion of CO2 into usable products, further research is urgently needed. The aim is to improve the efficiency and practical applicability of these processes. The ability to effectively convert CO2 into valuable resources is central to the fight against climate change. Further studies could help not only to reduce atmospheric CO2 levels, but also to develop innovative materials for industrial applications. This research is at the beginning of a promising development in CO2 reduction technology, which has the potential to both reduce the burden on the environment and open up new avenues in materials science.
Author: Lasse S. Martinsen

Investing in real estate with vision: How the Dr Barbara Mez-Starck Foundation creates sustainable added value
The decision of the Dr Barbara Mez-Starck Foundation to invest in the construction of real estate marks a turning point in the Foundation's history and underlines its commitment to the promotion of science and education. This investment goes far beyond the financial aspect and contributes in many ways to the fulfilment of the Foundation's purpose.
Supporting science and education
With the construction of the Mez-Starck House, the Foundation has not only made a secure capital investment, but has also created a place that supports research and education on several levels. Senator Werner Braun emphasises the importance of this investment: "By investing in a sustainable property on the campus of Ulm University, we are not only making a smart investment, but also promoting scientific progress".
The building will provide the Institute of Theoretical Chemistry and its Chemical Information Systems group with a modern and efficient working environment, which will have a positive impact on their research in the natural sciences. "This building will not only improve the working conditions for us scientists, but will also be a magnet for young talent," says Prof. Dr. Axel Groß, emphasising the importance of this facility for the scientific community.
Sustainable and long-term financial security
By leasing the building to the University of Ulm, the Foundation not only secures long-term income, but also strengthens the bond between the Foundation and the university. This underlines the Foundation's commitment to making a long-term contribution to Ulm as a centre of science.
An architectural concept with a vision
The Mez-Starck House on Oberer Eselsberg, designed by architect Stefan Rapp, is a prime example of forward-looking construction. "Our aim was to create a building that is not only functional and aesthetically pleasing, but also takes ecological aspects into account," explains architect Rapp. By using wood and concrete in a hybrid construction method, a sustainable and resource-conserving architecture was created that emphasises the circular idea and thus makes a significant contribution to protecting the environment.
A model investment for foundations
The investment of the Dr Barbara Mez-Starck Foundation in the construction of a building for the University of Ulm is an exemplary model for the combination of economic foresight and ecological responsibility. The Mez-Starck House stands not only for sustainable architecture and the promotion of science, but also for the visionary strategy of a foundation that knows how to fulfil its purpose in an innovative way. The Dr Barbara Mez-Starck Foundation thus sets standards for future-oriented commitment and emphasises the importance of investments that create both financial security and social added value.

Chances of success for Cluster of Excellence increase for the University of Ulm
Chem4Quant prevails over other applicants
The University of Ulm has taken a significant step forward in its strategic partnership with the renowned Karlsruhe Institute of Technology (KIT) and the University of Stuttgart by submitting a joint application for a prestigious Cluster of Excellence. This application marks an important milestone in the University's efforts to further expand and strengthen its research capacities and competencies.
As part of this ambitious project, the consortium has now overcome a major hurdle: The "Chem4Quant" initiative from the University of Ulm has succeeded in the highly competitive selection process against a large number of submissions - 143 outlines to be precise. Only 41 of the outlines were selected for the next stage of the application process - the submission of a full proposal. This success not only underlines the high quality and innovative strength of the proposed research ideas, but also puts the Chem4Quant initiative in an excellent position in the race for the coveted funding. If the full application is successful, the initiative will join an exclusive group of 98 Clusters of Excellence that have applied for funding, including the 57 clusters that have already been funded since 2019.
The "Chem4Quant" Cluster of Excellence is at the heart of scientific progress in unexplored areas of quantum technology. It will develop material structures with microscopic precision that will serve as the basis for the most advanced quantum technologies of the future. Experts from the fields of computer science, materials science, physics and chemistry are pooling their expertise to generate new knowledge through interdisciplinary collaboration.
"Chem4Quant has a strong network of established collaborations and thus a unique expertise in the field of molecular quantum systems. For example, chemically definable quantum architectures serve as the basis for a future quantum internet. On a chemical basis, qubit materials are designed to meet the special requirements of quantum technology.
This approach is so special because many of the chemical platforms currently in use are limited in terms of precise definition, scalability, positioning options and error compensation. For the first time, it would be possible to position electrical or photonic modules below the nanometre range. By exploring innovative qubit materials, the first elements of the future quantum internet can be realised.
This pioneering role in nanotechnology marks a turning point towards an era in which quantum communication overcomes the limits of theoretical models and can be implemented in practice. The ambitious Chem4Quant research project aims to provide a robust foundation for the realisation of a scalable and precisely controllable quantum infrastructure. Such an infrastructure is essential for the integration of quantum technologies into commercial applications. Overcoming current limitations in precision chemistry not only opens up innovative prospects for quantum computing, but also paves the way for the development of quantum communication systems that could have a profound impact on our information society. Every scientific achievement made within Chem4Quant will therefore contribute significantly to the transformation of quantum technology from a fascinating theoretical possibility into a practical, advanced technology.
With the Chem4Quant and POLiS initiatives, the University of Ulm is now positioning itself with two promising applications in the competition for the coveted excellence clusters. The POLiS (Post-Lithium Energy Storage) project has been funded since 2019 and is a prime example of the outstanding research being carried out at the university. At the heart of the initiative is the University of Ulm's renowned Institute of Theoretical Chemistry, which is supported by the Dr Barbara Mez-Starck Foundation. Prof. Dr. Axel Groß, Director of the Institute, plays a central role as one of the leading spokespersons of the cluster and has been instrumental in preparing the continuation application, which will be submitted in cooperation with the Karlsruhe Institute of Technology (KIT) and the Justus Liebig University of Giessen.
The commitment and enthusiasm for research of the scientists involved give the Institute an important role in this network. At the same time, they reflect the Institute's deep roots in the principles of academic excellence. The Chem4Quant and POLiS projects also open up valuable synergy effects, particularly in materials-oriented basic research, which have the potential to have a lasting impact on the research landscape.
The approval of these two cluster proposals would enable the University of Ulm to take a further important step within the framework of the Excellence Strategy of the German federal and state governments: to apply for the "University of Excellence" funding line. Such a development could significantly increase the visibility and recognition of Ulm University's research activities. Being recognised as a University of Excellence would not only be a confirmation of the work done so far, but also an important catalyst for future research initiatives. It would enable the University to gain even greater national and international recognition as a leading research institution and to further consolidate its position at the forefront of scientific innovation and excellence.
No decision on future clusters of excellence until 2025
The Cluster of Excellence funding programme is part of the Excellence Strategy of the German federal and state governments and is implemented by the German Research Foundation (DFG). The selected Clusters of Excellence receive annual funding of three to ten million euros for seven years in each case. A maximum of two funding periods totalling 14 years are possible. The final funding decision on the future Clusters of Excellence will be made at the end of May 2015.

Interview with Prof. Dr. Melanie Schnell
Hello Prof. Dr. Melanie Schnell, thank you for taking the time to give us an interview. If one googles you, you quickly get a first impression of the topics you have already researched in your field and, above all, where you have travelled around the world. We are very impressed and find the subject of your research very exciting, even though we have to admit that we understand very little about it from a technical point of view.
At what point in your life did you first become interested in research, and in physics and chemistry in particular?
I became interested in research right at the beginning of my studies. I found and still find it fascinating to acquire basic knowledge and then work on developing it further.
I grew up in a village and very close to nature. My grandmother had a very large vegetable garden. From an early age I wondered why, for example, some plants grew from "onions", others from "beans" and still others from small seeds. With this early curiosity for such more biological connections, I discovered my interest in chemistry at school, until I ended up studying physical chemistry - ideal for me: Developing physical methods to understand chemical relationships. Again, my childhood came back to me, and still does. My father built many things at home - and we children were allowed and (sometimes) obliged to help. I was introduced to tools and manual work at an early age - an important basis for our laboratory work.
Could you briefly explain what your area of research represents and where your results can be used?
I'm not very good at summarising, so here's a more detailed short version.
We develop and use spectroscopic methods to analyse molecular compounds with high precision. Our research interest is at the molecular level - for example, we want to understand in detail how molecules interact with each other and how even the smallest changes to these molecules can lead to other interactions. Ultimately, it is these interactions that determine the function and therefore the efficacy of molecular compounds. For example, even relatively small molecules can change their preferred structure depending on whether they are in water or ethanol, i.e. alcohol, as a solvent. This needs to be understood.
A second focus of our research is the study of chiral molecules. These molecules exist in two forms, the enantiomers, which, like our two hands, are mirror images, i.e. they cannot be easily converted into each other. While these two enantiomers have identical physical properties, such as boiling and melting points, they can have very different biochemical properties. This can be seen, for example, in the different efficacy of chiral drugs such as ibuprofen: While one enantiomer of ibuprofen has the well-known analgesic and anti-inflammatory effect, the other enantiomer is initially largely ineffective and is eventually converted by an enzyme in the body into the active component - an interesting long-term effect. These differences in biochemical and medical efficacy need to be understood at the molecular level. We are also developing methods to identify and distinguish chiral molecular compounds.
Our third area of research takes us off the Earth and into space. We are characterising molecular compounds that may also play a role in the chemistry of interstellar space. This space between the stars is not empty, but filled with molecular clouds, which may continue to condense and become the beginning of a new star. The inventory of such molecular clouds is studied with telescopes. These telescopes can collect the molecular fingerprints that the molecular compounds emit after being excited by starlight. To be able to match these spectral fingerprints to specific molecular compounds, we study suitable molecular compounds in the laboratory and provide this information to the radio astronomers operating the telescopes for their analyses. In this way, you can gradually solve the big puzzle of which molecular compounds exist in the universe, how they were formed and how they might react further.
You have been a professor at Kiel University for several years and a senior scientist at DESY in the "Spectroscopy of Molecular Processes" research group. What are you currently researching in particular? What can we look forward to?
I have already mentioned some general examples of our research. For example, we are currently working on not only distinguishing the enantiomers of chiral molecules, but also separating them so that we can then carry out very controlled studies on them. Our method is particularly interesting for molecular systems whose enantiomers cannot be easily separated by other means. This is where we come in.
What made you decide to get involved with the Dr Barbara Mez-Starck Foundation? How did you find out about the foundation?
I have known about the Dr Barbara Mez-Starck Foundation for many years, mainly through the Mogadoc database, which we used during my PhD studies at the University of Hanover to quickly access spectroscopic information on certain molecular compounds. In my field of research, molecular spectroscopy, the Dr Barbara Mez-Starck Foundation is also known beyond national borders through the international Dr Barbara Mez-Starck Research Prize, and I felt very honoured when I was awarded this prize in 2021.
You have already won numerous prizes and awards for your research, including the Helene Lange Prize in 2013, which is awarded exclusively to young female scientists in the STEM subjects and honours their outstanding achievements. How do you see the gender imbalance in science?
First of all, it is a general concern of mine to get young people interested in science, for example through lectures, internships in the research group or visits by schoolchildren.
I also firmly believe that role models are very important in helping people to imagine and have confidence in certain career and development paths. I try to be very attentive to this, for example by making sure that there is a good balance of female and male scientists at the conferences we are planning. This increases the visibility of individuals, but also the visibility of women scientists in general. When I studied chemistry at the end of the 1990s, there was not a single female professor of the subject at my university. I didn't really notice this at first. It was only when I changed universities that I realised that it was motivating for me to work with female professors - role models. Diversity is important to keep everyone open to different views and ideas. Especially in times when some people in public positions promise supposedly simple solutions, this becomes more and more important.
Finally, the burning question: Are you also active on social media channels and if so, with what intention and on which platforms?
No, as a research group we are not active on social media channels. We have an active website and do a lot of outreach work, e.g. with schools as mentioned above, but no social media activities.
Thank you very much for your time and we look forward to hearing more from you from the Dr Barbara Mez-Starck Foundation in the future.

How the discoveries of theoretical scientist Dr Sotoudehs are changing the battery world.
An interview about the future of energy storage
Lasse Martinsen: Dr Mohsen Sotoudeh, as a leading theoretical scientist in the field of biochemistry, you are talking to us today about your work and the future of battery technology. Could you start by telling us a little bit about your career and your current work?
Dr Mohsen Sotoudeh: Of course, Mr Martinsen, and thank you very much for the invitation. I have been working in the field of materials science, theoretical physics and chemistry for over a decade, with a particular focus on battery technologies and electrochemical storage systems. I am currently working on my habilitation thesis at the University of Ulm, focusing on the development of new materials for more powerful and sustainable batteries.
Lasse Martinsen: That sounds like exciting and forward-looking work. Can you give us an example of a discovery that you are particularly proud of?
Dr Mohsen Sotoudeh: One of the highlights of my career was the development of a so-called ion mobility descriptor, which shows a strong correlation between the activation energy for diffusion events and the ionic conductivity of materials. This work enabled us to understand and predict complex properties such as ion migration using simple physical parameters such as electronegativity and ion radii. What was unique about this approach was that we were able to identify materials that were particularly suitable for use in batteries without actually having to manufacture and test them. This discovery, which we made by introducing the concept of binding characteristics using electronegativity, was a major step forward in materials science and was confirmed by a linear scaling relationship. I am proud of this achievement as it paves the way for rapid and efficient discovery of new materials for battery applications.
Lasse Martinsen: You mention that it takes time for scientific discoveries to be translated into practical applications. How do you deal with the impatience of society, which is often looking for immediate solutions?
Dr Mohsen Sotoudeh: This is a challenge that many scientists face. It is important to strike a balance between enthusiasm for the potential of new discoveries and the patience required to carefully validate and develop these discoveries. We try to be transparent about what is feasible and what needs more time to avoid misunderstandings.
Lasse Martinsen: In addition to your research, you also seem to have a strong focus on collaboration, both within your discipline and beyond. Could you tell us more about this?
Dr Mohsen Sotoudeh: Yes, interdisciplinary cooperation is crucial. In my work, I meet colleagues from physics, chemistry, materials science and even engineering. This diversity of perspectives enriches our research enormously. I also value working with the next generation of scientists. They bring fresh ideas and new approaches that are essential for solving the complex challenges we face.
Lasse Martinsen: Do you recognise the importance of transparency and openness in science and how does this affect your work?
Dr Mohsen Sotoudeh: Transparency not only promotes trust in science, but also accelerates the acquisition of knowledge. By sharing data, methods and results, we can avoid duplication and build on the results of others. This is particularly important in my field of battery research, where rapid progress is urgently needed to address global energy challenges.
Lasse Martinsen: What advice would you give to students or young researchers who want to pursue a career in your field of research?
Dr Mohsen Sotoudeh: I would tell them to stay curious and cultivate their passion for science. It is important not to limit yourself to a narrow specialisation, but also to develop interdisciplinary skills. The world of battery technology and materials science is incredibly diverse and offers many opportunities for innovative research. You should also not underestimate the importance of communication and collaboration. The ability to communicate ideas clearly and collaborate with other disciplines is crucial to scientific progress.
Lasse Martinsen: As a researcher, don't you also have concerns about the theft of your ideas when you put so much emphasis on transparency?
Dr Mohsen Sotoudeh: I am convinced that transparency is really the key to protecting and clearly attributing ideas. The more openly and freely ideas are shared, the easier it is to trace who contributed the original approach.
Lasse Martinsen: You decided in favour of the University of Ulm a few years ago. What was the main reason for moving here, especially with regard to your research in battery technology?
Dr Mohsen Sotoudeh: The main reason for moving to the University of Ulm was the excellent research infrastructure and the university's strong commitment to battery technology and materials science. Ulm is known for its leading role in these areas, supported by close cooperation with industrial partners and other research institutions. In particular, funding from the German Research Foundation (DFG), the Mez Starck Foundation and participation in various interdisciplinary and international projects provide ideal conditions for forward-looking research. I also had the opportunity to work with leading experts in the field of battery research, which was a decisive factor in my decision to come to Ulm. The combination of an excellent research environment, support for innovative projects and an open, collaborative atmosphere made Ulm the ideal place for my scientific career and development.
Lasse Martinsen: How do you see Germany's role in global battery research and technology?
Dr Mohsen Sotoudeh: Germany has a strong tradition of research and development, especially in engineering and chemistry, which provides a solid foundation for innovation in battery technology. However, there is still some catching up to do internationally, especially with countries such as China and the US, which are investing heavily in battery research. Germany has the potential to play a leading role, particularly by promoting research initiatives and cooperation between universities, foundations, research institutes and industry. The recent investments in battery research centres and the focus on developing sustainable battery technologies are positive steps in this direction.
Lasse Martinsen: What specific challenges do you see in developing the next generation of batteries?
Dr Mohsen Sotoudeh: One of the biggest challenges is the balance between energy capacity, lifetime and safety of batteries. We need to find materials that not only enable high energy density, but are also stable, safe and environmentally friendly. Another important issue is sustainability, in particular reducing dependence on rare or harmful materials and improving the recyclability of batteries. Finally, the challenge is to scale up production of new battery technologies to make them economically viable and widely available.
Lasse Martinsen: What inspires you outside of science? Do you have any hobbies or interests that give you new perspectives or a break from the daily lab work?
Dr Mohsen Sotoudeh: Outside the lab, I find relaxation and inspiration in a lunchtime walk with my colleagues. It gives me a chance to switch off and think about my work from a different perspective. Swimming is also an important part of my life. Not only does it help me relax, but it also develops my creativity and problem-solving skills, which I can use in my scientific work.
Lasse Martinsen: Finally, Dr Sotoudeh, what motivates you personally in your work and what are your hopes for the future of battery technology?
Dr Mohsen Sotoudeh: My biggest motivation is the prospect of contributing to a more sustainable and energy efficient future. Battery technology is a key area that can revolutionise the way we store and use energy. I hope that through our research we can contribute to more efficient, safer and more environmentally friendly battery systems. These systems could not only transform mobility, but also improve access to clean energy around the world. Ultimately, I dream of a world where clean energy is accessible to all and where scientific innovation goes hand in hand with protecting our planet.
Lasse Martinsen: That is an inspiring vision, Dr Sotoudeh. Thank you for sharing your insights and hopes with us. Your work and commitment to science and a better future are truly admirable. We wish you continued success with your research.
Dr Mohsen Sotoudeh: Thank you very much, Mr Martinsen. It was a pleasure to be here and talk about my passion. I hope that our discussions will help to make more people aware of the importance of science and, in particular, of developments in the field of battery technology. Let's work together towards a more sustainable future.

The future of battery technology: Prof Dr Axel Groß discusses the role of quantum chemistry in materials development at Battery Forum Germany 2024
The annual Battery Forum Germany congress took place in Berlin from 24 to 26 January 2024. Scientists from research and development institutions and industry networked and exchanged ideas. This year's three-day congress focused on methods for accelerating cell development, battery cell components, simulation and machine learning, second use, repair and remanufacturing as well as battery systems.
Prof Dr Axel Groß, Head of the Institute of Theoretical Chemistry at the University of Ulm, made an important contribution to the discussion on current scientific and technical topics with his invited lecture (Possible) contributions of quantum chemistry to accelerated material development in batteries. The presentation focused on the improvement of the understanding of atomic processes at surfaces, interfaces and in battery materials, based on the theoretical description of these processes using the fundamental principles of quantum chemistry, and the ability to elucidate structures and processes in batteries through microscopic mechanisms.
The approach of using oxide perovskites as the cathode material was of particular interest to the specialist audience, as it offers a way to use less critical materials than in lithium-ion batteries, thus providing an approach to the development of relevant alternative battery technologies.
"The coming together of leading minds from science and industry is at the heart of our progress," emphasised Prof. Dr Axel Groß. "This kind of exchange is essential in order to find innovative solutions to the challenges of our time. I am impressed by the interest and commitment of the audience here at the Battery Forum. Only as a community can we pave the way for pioneering technologies."
Indeed, the Battery Forum Germany is a central hub that goes far beyond the presentation of new research. The event promotes the critical dialogue and creative collaborations that are essential to pushing the boundaries of battery technology. By bringing together a wide range of expertise, the Forum is turning into a source of inspiration, enabling participants not only to share their ideas, but also to develop them and turn them into concrete, interdisciplinary projects.
The congress, now in its twelfth year, is an ideal platform for a comprehensive exchange on battery technology - covering the spectrum from scientific findings to economic aspects and political perspectives. In addition to the 300 or so participants in Berlin, there was also an online audience. The conference was organised by the Competence Network Lithium-Ion Batteries (KLiB) with the support of the German Federal Ministry of Education and Research (BMBF).
The pioneering research work of the Institute of Theoretical Chemistry at the University of Ulm receives significant support from the Dr Barbara Mez-Starck Foundation. This funding enables the institute to be at the forefront of scientific research and development in the field of electrochemical energy storage and to make significant progress in accelerating the development of materials for battery technologies.
Author
Kaja G. Martinsen


A step towards improved battery technologies
When magnesium (Mg) meets chloride
An innovative technology is attracting a lot of attention in the world of electrochemistry: Chloride-ion batteries (CIBs). This innovative development could provide a low-cost and sustainable alternative to the way we store and use energy. This is because it is based on elements that are very common and therefore promise high security of supply and low prices. At the heart of this technological advance is the interaction between halides, especially chloride and metal anodes. Halides are the characteristic anions in electrolytes and their role in batteries goes far beyond pure conductivity. They are largely responsible for the efficiency and performance of batteries through their strong reaction with metal anodes. Magnesium anodes in particular are the focus of current research as they offer new opportunities for the development of high performance energy storage systems. This important research at the University of Ulm is made possible by the funding and support of the "Dr Barbara Merz-Starck Foundation".
Magnesium and chloride: A promising combination
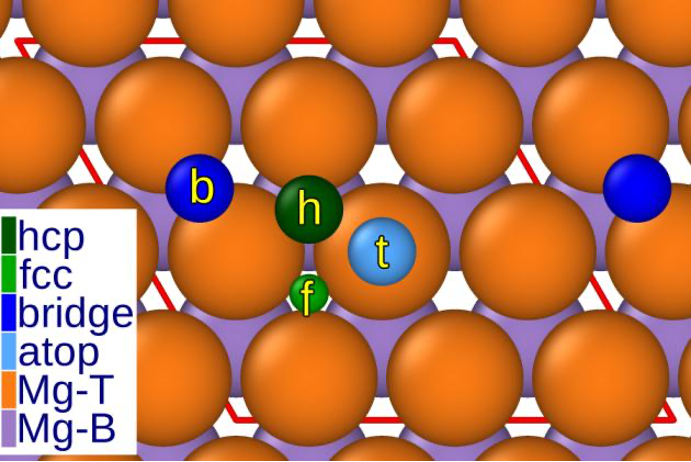
The interaction between Mg and chloride is attracting a lot of attention in the scientific community, particularly because of the potential use of Mg in CIBs. Despite earlier concerns about the susceptibility of Mg to corrosion by chloride, studies have produced interesting results. It was found that chloride ions prefer specific sites on the magnesium surface, especially in hexagonal close-packed (hcp) and face-centred cubic (fcc) cavities.

Fig. 1 Various adsorption sites on the magnesium surface. "Hollow" site at hcp and fcc, which can be regarded as favoured adsorption positions.
Salt layers and surface structure
Another area of research concerns the formation of salt layers on metals, a phenomenon that is similar to rust formation. These salt layers could affect the performance of magnesium batteries. The study examines how chlorine (Cl) attaches to Mg, a material characterised by a stable surface. Interestingly, the distance between the atomic layers on the magnesium surface changes in a way that differs from most metals.
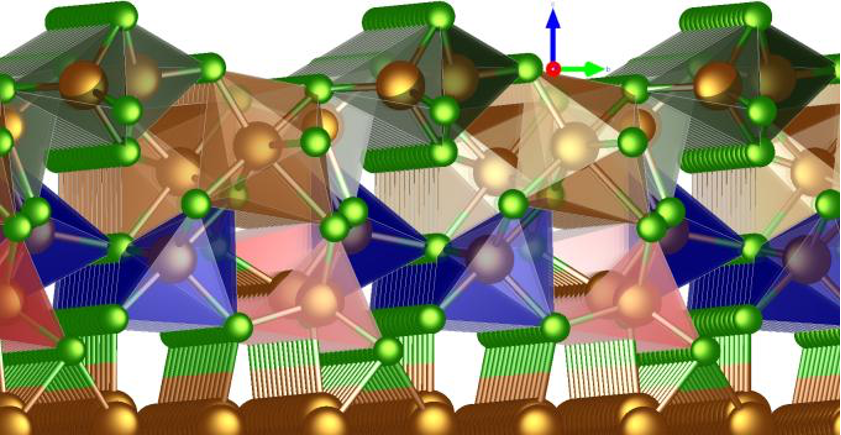
Fig. 2 Illustration of the most stable Cl adsorbate structure
New perspectives through salt layer research
By using techniques such as "adaptive random mutation hill climbing", based on evolutionary computing, scientists at the Institute of Theoretical Chemistry at the University of Ulm were able to determine the optimal arrangement of chlorine and magnesium atoms at higher chlorine coverages. These investigations show that the surface structure of Mg changes significantly as the number of chlorine atoms increases, which could lead to the formation of a more open and complex structure. This knowledge is crucial for understanding the stability of magnesium surfaces.
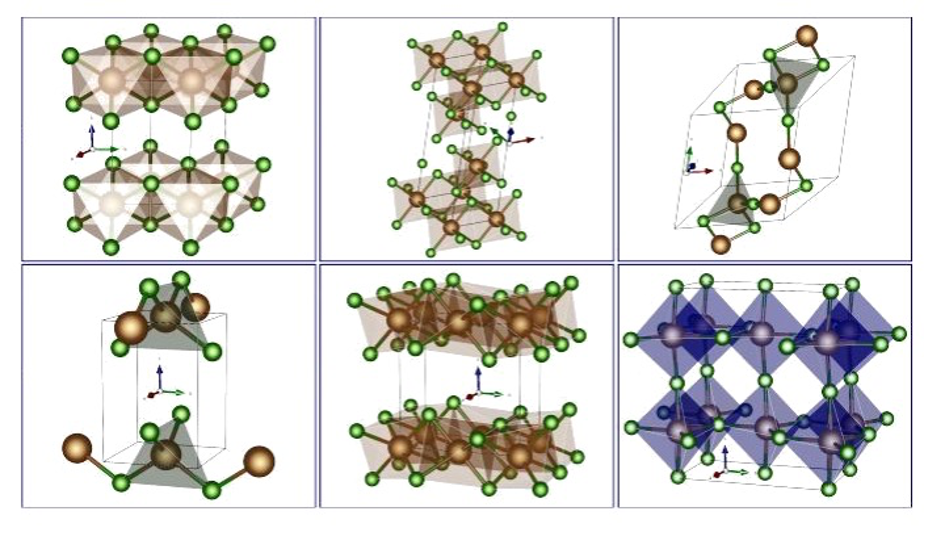
Fig. 3 Illustration of differently stable MgCl2 salt structures
New findings on salt layer formation on Mg
The detailed study of the salt layer by Professor Dr Axel Groß and his team in Ulm, Germany, revealed that this layer consists of a large number of different magnesium chloride structures. This result is particularly revealing as it indicates that the magnesium surface undergoes a direct change when chloride ions are added - a crucial process in batteries during energy release.
Implications for future battery technologies
The discovery that chloride ions directly induce the formation of a salt layer on the magnesium surface represents an innovative breakthrough. This finding could make a significant contribution to improving and increasing the efficiency of future battery technologies. The research results presented by the University of Ulm broadens the field of energy storage technologies and lays the foundation for further studies aimed at developing more sustainable and higher-performance storage solutions.
Source
1. Sarkar K, Hübner D, Stottmeister D, Gross A. Unraveling the Intricacies of Surface Salt Formation on Mg(0001): Implications for Chloride-Ion Batteries. ChemRxiv. 2023; doi:10.26434/chemrxiv-2023-hs8t9
This content is a preprint and has not been peer-reviewed.
Author
Lasse S. Martinsen

Oxide perovskites - pioneering materials for the battery technology of the future?
Ulm University paves the way for sustainable electric mobility with innovative
post-lithium battery solutions
The electrification of the transport sector is a key step towards achieving the climate targets. Lithium-ion batteries are currently the dominant technology in electric vehicles due to their high energy density and performance. However, they face challenges such as growing demand, which is leading to increasing scarcity, and the environmental impact of mining and processing these materials. As a result, there is intense research into alternative battery technologies based on less critical materials. One promising approach is to use oxide perovskites as the cathode material. Oxide perovskites are materials with a characteristic crystal structure that are flexible and easy to process. They can be doped with different metal ions to achieve the desired properties.
Oxide perovskites as an alternative
Oxide perovskites as a cathode material for post-lithium batteries are a promising alternative to lithium-ion batteries because they are easy to synthesise. They also have high specific energies and energy densities and are generally considered to be more environmentally friendly. As part of a computer-aided study at the University of Ulm, led by M.Sc. Johannes Döhn and Prof Dr Axel Groß, 280 different oxide perovskites were investigated for their suitability as cathode materials for post-lithium batteries. The specific energy, energy density and other relevant properties of these compounds were calculated using density functional theory (DFT)
Of the 280 compounds, 30 passed the initial screening process. A further 17 were discarded because the perovskite structure collapsed during the DFT geometry optimisation, leaving only 13 compounds. Figure 1 shows a cathode material for batteries based on a perovskite structure, as analysed in this study. The material ABO3 represents the state at low charge and consists of a complete intercalation of components (shown here in blue) in the lattice. In contrast, BO3 shows the material in a charged state, characterised by the removal of these components.

The investigation of the remaining 13 compounds was based on the diffusion barriers for the migration of the shuttle ions, a critical factor for battery performance. For example, poorer ion migration at low temperatures is the main reason for the reduced range of battery-powered vehicles in winter. The selected materials and their specific diffusion pathways are shown in Figure 2, which also shows the diffusion barriers of the 13 successfully screened compounds. It is particularly noteworthy that MgNbO3, ZnVO3 and AlMoO3 show low values at the upper vacancy limit, indicating high ionic mobility.
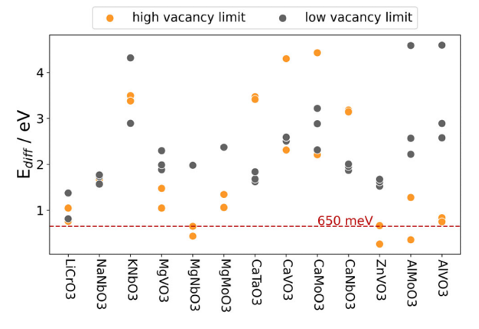
The final selection included MgNbO3, ZnVO3 and AlMoO3, with MgNbO3 standing out in particular.
MgNbO3: The most promising candidate
MgNbO3 was identified as a particularly promising candidate for applications. Figure 3 shows a detailed analysis of the diffusion path of MgNbO3, with the diffusion path running downwards in the intercalated material. This indicates an energetically favourable intermediate configuration rather than a final configuration. Examination of individual structural images shows that the characteristic corner-linked oxygen octahedrons of the perovskite structure are preserved throughout, ensuring structural integrity and suggesting no collapse of the structure. This suggests that the effective energy barrier for solid-state diffusion may be lower than originally thought, allowing particles to move more easily than expected.
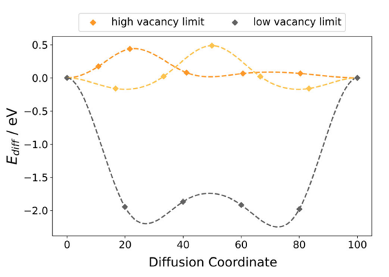
Everyday relevance
The results of the study, which was co-financed by the MEZ Starck Foundation, have the potential to have a significant impact on everyday mobility. By improving the range and performance of electric vehicles, these technologies could help to reduce operating costs. This would make electric vehicles affordable to a wider range of buyers, which could have a positive impact on reducing CO2 emissions. On an individual level, progress means that consumers could have access to electric vehicles with better performance and greater range, making electric vehicles a more viable alternative for personal transport. At a societal level, the introduction of this new battery technology could reduce dependence on lithium and other critical raw materials, thereby reducing the environmental impact of their extraction and processing. In short, the use of oxide perovskites in batteries could have far-reaching positive consequences for individuals and society as a whole, from financial savings to environmental protection.
Looking to the future
Ulm University’s research represents a significant step forward in the development of more sustainable and efficient post-lithium batteries. The study lays the groundwork for optimising the synthesis of MgNbO3, a promising material for future battery technologies. Further research is needed to confirm the theoretical properties in practice. Future studies, in collaboration with the MEZ Starck Foundation, could focus not only on improving the production of MgNbO3, but also on increasing the lifetime of oxide perovskite-based batteries. The aim is also to develop new oxide perovskite compounds with even higher specific energies and energy densities.
In the coming years, the rapidly developing field of oxide perovskite research could make a decisive contribution to the realisation of batteries that are not only more powerful and durable, but also have a lower environmental impact. The advantages of oxide perovskites, such as high specific energy and energy density, make them a key material for the sustainable battery technology of the future. The current research results provide a solid basis for the commercial use of oxide perovskite batteries, but further research will be crucial to realise the full potential of this material and confirm it in real applications.
Source:
Johannes Döhn and Axel Groß, Computational Screening of Oxide Perovskites as Insertion-Type Cathode Material, Adv. Energy Sustainability Res. 2300204 (2023), Open Access, DOI: 10.1002/aesr.202300204, posted on ChemRxiv, DOI: 10.26434/chemrxiv-2023-vj973 [Paper-Link]
Author:
Lasse S. Martinsen

At the last board meeting of the Dr. Barbara Mez-Starck-Foundation on November 24, 2023, two new advisory boards were appointed. Together with us, they will contribute to the advancement of all issues of the Foundation and support the board accordingly.
Prof. Dr. Melanie Schnell
professor for physical chemistry
Deutsches Elektronen-Synchrotron DESY Hamburg
and Universität zu Kiel
Hamburg
Volker Herrdum-Heinrich
former bank director
Freiburg

On October 19, 2023 the local Dr. Barbara Mez-Starck-Prizes for the best Master-graduates were awarded for the academic year 2021/2022 in a special awarding ceremony. Four outstanding Master-graduates in Chemistry were honoured.

On October 27, 2022 the Dr. Barbara Mez-Starck House was inaugurated. Despite of the corona-pandemic and the Ukraine crisis the ecologically sustainable building was established within the given time frame and financial budget.

On August 29, 2021 Prof. Melanie Schnell (Deutsches Elektronen Synchrotron in Hamburg) and Prof. Stephan Schlemmer (University of Cologne) were awarded with the international Dr. Barbara Mez-Starck Prize.
Dr. Barbara Mez-Starck-Stiftung
Commerzbank AG
Nachlass- und Stiftungsmanagement
Kaiserstr. 16
60311 Frankfurt am Main
Telephone +49 (0) 69 – 13653934
Telefax +49 (0) 69 – 13650269
PC fax +49 (0) 69 – 405650056
E-mail: info[at]mez-starck-stiftung.de
